Integrated Services
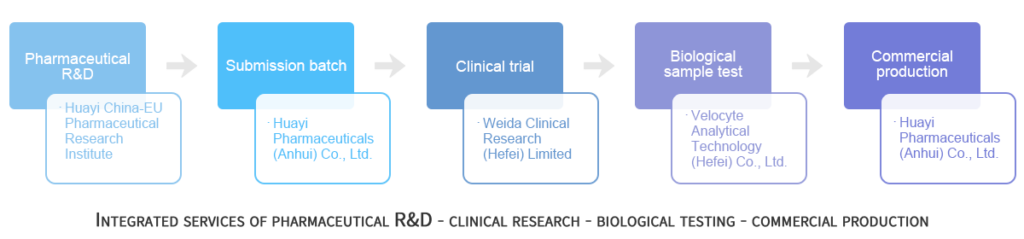
Huayi Pharmaceuticals offers an integrated service for product development with our partners Weida Clinical Research and Velocyte Analytical Technology for your bioequivalence needs in China. Through these close strategic partnerships, we are able to offer a one-stop service which allows pharmaceutical development, dissolution profiling, stability studies, bio-equivalence studies, testing of plasma samples and commercial manufacture. Having these integrated services ensures the success of your projects, shortens the time taken for R&D and keeps your project cost-effective. The integrated service is particularly attractive for clients that want to develop products for the Chinese domestic market or for both the Chinese and European markets.
Why become our partner?
Since our establishment, we have completed site transfers of over 50 products and supply a billion doses a year to European customers. We have successfully developed dossiers for Europe resulting in granted marketing authorisations.
We have recently expanded our capability to develop products to Chinese standards also and can produce dossiers that meet both European and Chinese requirements. Together with our associated company Velocyte, we can also offer bio-equivalence studies in China to seamlessly complete the clinical part of the development.
Huayi is committed to upgrading and developing our services. We aim to increase our current manufacturing capacity from 3 billion units to 5 billion units to satisfy demand. We have built new facilities for high-potency and oral liquids manufacturing and put in to use in 2021.
API characterisation
Analytical method development
Formulation development
Scale up
Validation
Bio-equivalence studies in China through our partner company
Contract manufacturing and commercial supply
Full regulatory compliance (toxicological assessment, serialisation etc.)
Research & Development
We are experienced in technology transfers and scale-up process for commercial production. Having transferred over 50 products for our customers and registering almost 100 strengths to date, we are prepared to take on new challenges in pharmaceutical development with our experienced team from Huayi China-Europe Pharmaceutical Research Institute.
Read More01 / 04
Quality Management
Huayi’s QA team is committed to ensuring your project adheres to EU cGMP standards at every step of production from development to commercial manufacture. We will work hard to ensure that your project will be completed on time to the highest quality and stay focused and communicative throughout the process.
Read More02 / 04
Contract Manufacturing
In 2018, our contract manufacturing output reached 1.6 billion units. Huayi manufactures solid oral dosage forms including film-coated, sugar-coated, enteric-coated, effervescent, soluble, chewable and buccal tablets as well as capsules. Our high-potency and liquid preparation facilities will be ready in 2020.
Read More03 / 04
Integrated Services
We offer bioequivalence studies for your next project in China. Through our partners Weida Clinical Research and Velocyte Analytical Technology, we can conduct clinical trials, biological sample testing, dissolution profiling and stability studies. We can then scale-up from the test batch to commercial production.
Read More04 / 04




